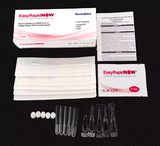
Rapid Detection AntIgen Test (5 Kits)
- Included:5 Test Cassettes
- 5 Nasal Swabs
- 20 Pipettes
- 5 Individual Buffers
- 5 Alcohol Prep Pads
EasyRapidNow™ COVID-19 Antigen Rapid Test Kit is not yet approved by the US FDA.
We have submitted all documentation and are awaiting FDA EUA approval.
These tests are available for SURVEILLANCE TEST PURPOSES only and are NOT intended for use in Diagnostic Procedures in the USA.
· This test has not been FDA cleared or approved;
· This test has been authorized by FDA under an EUA for use by authorized laboratories;
· This test has been authorized only for the presence of IgM and IgG antibodies against SARS-CoV-2, not for any other viruses or pathogens; and
· This test is only authorized for the duration of the declaration that circumstances exist justifying the authorization of emergency use of in vitro diagnostics for detection and/or diagnosis of COVID-19 under Section 564(b)(1) of the Federal Food, Drug and Cosmetic Act, 21 U.S.C. § 360bbb-3(b)(1), unless the authorization is terminated or revoked sooner.